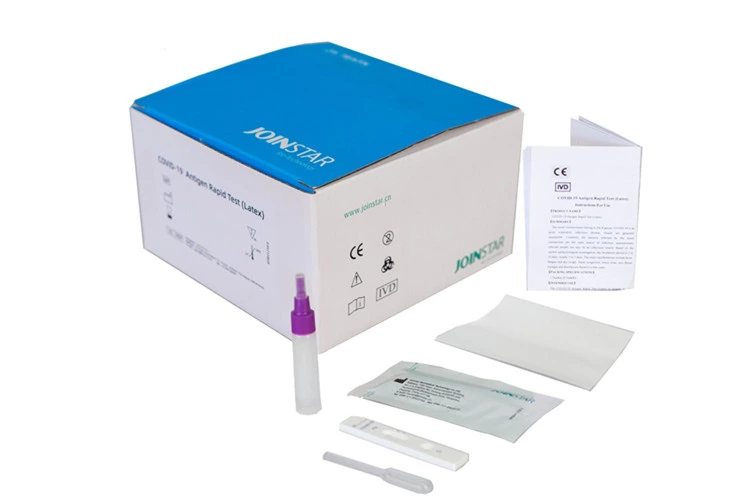
COVID-19 Antigen Rapid Test(Latex)
The Joinstar COVID-19 Antigen Rapid Test provides qualitative detection of SARS-CoV-2 in oropharyngeal saliva, sputum or stool. No invasive sample collection is required to perform the test and is therefore particularly suitable for children, the elderly and people with disabilities.
The sample collection tubes are already pre-filled with the correct amount of buffer solution. The user therefore saves the step of decanting the buffer solution. Since this step is eliminated, the test can also be performed with less potential for error. The Joinstar Antigen Saliva Test has no cross-reactivity and is sensitive to corona mutations. The test result is available after only 15 minutes.
COVID-19 Antigen Rapid Test Feature
uInnovative tests globally
uHighly accurate due to high affinity between ACE2 receptor and S protein
uSensitive to various mutants. None cross reactivity
uNoninvasive sampling with various types of samples(*oropharyngeal saliva/sputum/stool/)
uUnique flexibility with oropharyngeal Saliva /Sputum/Stool- It can be performed everywhere and anytime!
uStore at 2℃-30℃, easy transportation.
uRapid: Results in 10~15 minutes
uEasy to Use. No machines required. No-chilled transportation required
uHome testing, designated institutions (such as hospitals) testing, and screening before restoration of functions of enterprises and schools
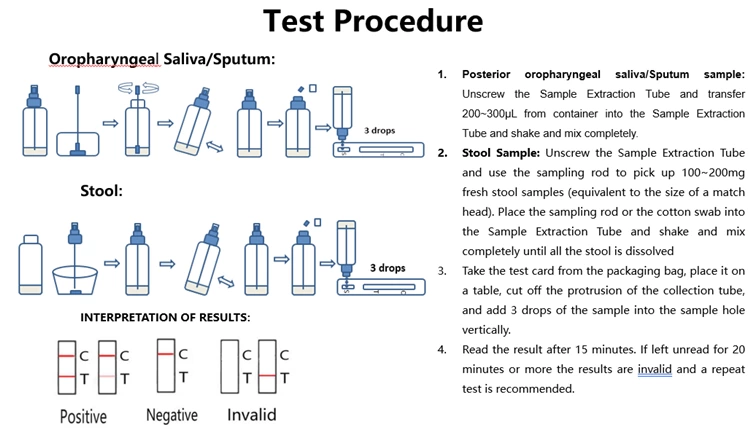

How does antigen rapid test work
The new coronavirus enters human cells through the specific binding of its spike glycoprotein (ligand) to the ACE2 receptor located on the human cell membrane. In this assay, the antibody was replaced by the ACE2 receptor to establish a new ligand-receptor chromatography test kit for rapid detection of the new coronavirus. In clinical practice, the test can be used for rapid detection of SARS-CoV-2 and all mutants. Testing is performed using posterior oropharyngeal saliva, sputum or stool samples from the subject. The test takes only 15 minutes and is much simpler and faster than nucleic acid testing (RT-PCR). The SARS-CoV-2 virus has been shown to evolve into contagious mutants through mutations in S1 proteins (such as D614G) that have stronger binding to ACE2 receptors. Given the current test format based on ACE-2 receptor binding, the test should also be able to detect such mutants.
The test kit contains a nitrocellulose membrane (NC membrane) onto which the rabbit anti-S1 protein of new coronavirus antibody is coated in the T-line region and the polyclonal goat anti-rabbit IgG antibody is coated in the control line region (C). Latex-labeled ACE2 protein and latex-labeled rabbit IgG are embedded in the reagent pad. To perform the assay, three drops of sample are added to the sample well, and the sample flows from the bottom to the top by capillary effect. After a 15-minute incubation, if the patient sample contains the virus, the latex-labeled ACE2 protein is bound by the S1 protein of the virus and then captured by anti-S1 protein antibodies coated on the TLine region. If the sample does not contain the virus, the latex-tagged ACE2 protein will not be captured by anti-S1 protein antibodies applied to the T-line region, therefore no T-line will appear. Regardless of whether the sample contains the virus or not, the latex-labeled rabbit IgG reacts with the polyclonal goat anti-rabbit IgG antibody coated on the control line region (C), and a colored line appears in the control region.
Once the assay is complete, the amount of latex ACE2 protein bound to the T-line is directly proportional to the
concentration of coronavirus in the sample, while the amount of latex bound to control line C does not correlate with the concentration of coronavirus bound to the T-line in the sample.
What is Sensitivity and Specificity of COVID-19 Antigen Rapid Test (Latex)
The COVID-19 Antigen Rapid Test (Latex) was compared with a leading commercial reagent (PCR). The results show that the COVID-19 Antigen Rapid Test (Latex) has high sensitivity and specificity.
Posterior oropharyngeal saliva specimen:
| Method | PCR | Overall result | ||
| COVID-19 Antigen rapid test (Latex) |
Results | Positive | Negative | |
| Positive | 54 | 0 | 54 | |
| Negative | 6 | 30 | 36 | |
| Overall result | 60 | 30 | 90 | |
Relative sensitivity: 90,00% (95% CI: 79,49% ~96,24%)
Relative specificity: 100,00% (95% CI: 88,43% ~100,00%)
Accuracy: 93,33% (95% CI: 86,05% ~97,51%)
Sputum sample:
| Method | PCR | Overall result | ||
| COVID-19 Antigen rapid test (Latex) |
Results | Positive | Negative | |
| Positive | 37 | 0 | 57 | |
| Negative | 3 | 30 | 33 | |
| Overall result | 60 | 30 | 90 | |
Relative sensitivity: 95,00% (95% CI: 86,08% ~98,96%)
Relative specificity: 100,00% (95% CI: 88,43% ~100,00%)
Accuracy: 96,67% (95% CI: 90,57% ~99,31%)
Stool test:
| Methode | PCR | Overall result | ||
| COVID-19 Antigen rapid test (Latex) |
Results | Positive | Negative | |
| Positive | 57 | 0 | 57 | |
| Negative | 3 | 30 | 33 | |
| Overall result | 60 | 30 | 90 | |
Relative sensitivity: 95,00% (95% CI: 86,08% ~98,96%)
Relative specificity: 100,00% (95% CI: 88,43% ~100,00%)
Accuracy: 96,67% (95% CI: 90,57% ~99,31%)